testing hardness of water gcse|hard water chemistry gcse : ODM Try this practical with your students to measure the hardness of water samples and investigate the effect of boiling. Includes kit list and safety instructions. 24 de jun. de 2021 · Origin. Fedposting has been a common part of 4chan board humor for over a decade, as the site often discusses things, such as guns and drugs, that would pique the interest of federal agencies. When .
{plog:ftitle_list}
WEBSinopse. A invenção de um país chamado Brasil começa em Portugal, onde o talentoso pintor Diogo Álvares (Selton Mello) cultiva a arte de embelezar a realidade, o que lhe cria alguns problemas com o comandante Vasco de Athayde (Luís Mello).
The properties of hard water have advantages and disadvantages. It is possible to measure the hardness of water, and distillation can be used to soften hard water. Part of Chemistry.Introduction. The amount of hardness in water can be determined using soap solution. Hardness is caused by calcium and magnesium ions. Hard water does not form a lather with soap as .Hard water Test questions. The properties of hard water have advantages and disadvantages. It is possible to measure the hardness of water, and distillation can be used to soften.Try this practical with your students to measure the hardness of water samples and investigate the effect of boiling. Includes kit list and safety instructions.
how to determine hardness in water
hardness of water gcse
A GCSE quiz focused on hard and soft water, looking at the causes and methods used to soften hard water. Also, looks at water quality and treatment.Markdown is as follows: **Hardness in Water**- Water hardness is primarily due to the presence of **calcium** and **magnesium** ions. - There are two types of hardness: **temporary .
Everything you need to know about Hardness in Water for the GCSE Chemistry (Triple) WJEC exam, totally free, with assessment questions, text & videos.
Water hardness is determined by the level of certain metallic ions like calcium and magnesium in the water. Hard water contains high concentrations of calcium and magnesium ions whereas .
What is the Hardness of Water? Water is said to be hard if it doesn't form a lather (bubbles) with soap. Calcium and magnesium ions cause water to be hard. Hardness is described as .This initial demonstration produces a range of different samples which students can test for hardness using soap solution, before finally investigating the effect of adding sodium carbonate. . Some hard water is poured into the tube above .Hardness in Water. Hardness in Water. Water hardness is primarily due to the presence of calcium and magnesium ions.; There are two types of hardness: temporary hardness and permanent hardness. Temporary hardness is caused by hydrogencarbonate ions (HCO3-) which can be removed by boiling.; Boiling precipitates the calcium and magnesium ions, effectively .
Water - Solubility - Ions. What is the Hardness of Water?. Water is said to be hard if it doesn't form a lather (bubbles) with soap. Calcium and magnesium ions cause water to be hard. Hardness is described as "permanent" or "temporary".. Rain water is naturally acidic due to dissolved carbon dioxide, (see the carbon cycle). The acid in rain water is carbonic acid, H 2 .
GCSE Chemistry (Triple) WJEC View topics (101) Topics. Acids, Bases and Salts TItration; Preparation of Crystals of Soluble Salts; . Hard water is water that contains high amounts of minerals, mainly calcium and magnesium ions. The hardness of water can be categorised into two types: temporary hardness and permanent hardness. .To ensure a fair test • use the same volume of each sample • use the same volume of soap • shake for the same length of time. 1. Temporary Hard Water: • Temporary hard water is caused by dissolved calcium hydrogencarbonate or magnesium hydrogencarbonate. • When the water is boiled, the hardness is removed as the hydrogencarbonate .Study with Quizlet and memorize flashcards containing terms like test for water and what colour it turns into?, highest daily volume of water?, lowest daily volume of water? and more. . GCSE Chemistry Water. Flashcards. Learn. Test. Match. Flashcards. Learn. Test. Match. Created by. Achsa_Santhosh. . testing hardness of water? GCSE workbooks https://www.amazon.co.uk/Dr-Shaun-Donnelly/e/B084FH9JPF?ref_=dbs_p_pbk_r00_abau_000000&_encoding=UTF8&tag=freesciencele-21&linkCode=ur2&linkId.
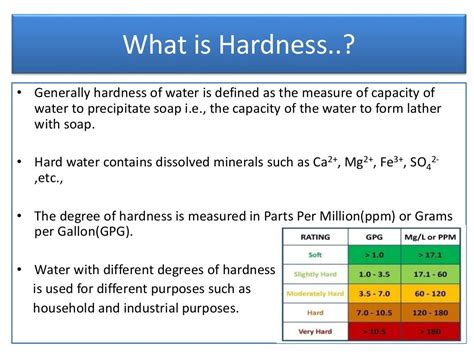
Electrolysing close electrolysis The decomposition (breakdown) of a compound using an electric current. water splits the water molecules (H 2 O) into hydrogen (H 2) and oxygen (O 2) molecules . To avoid the adverse effects of hard water, the calcium and magnesium mineral content in the water should be below 60 mg/L. The USGS standards for water hardness levels are as follows: Soft water: 0 to 60 mg/L; Moderately hard water: 61 to 120 mg/L; Hard water: 121 to 180 mg/L; Very hard water: Any level above 180 mg/L Check out my 2018 updated version of this video here: https://www.youtube.com/watch?v=dMKfNqSIdo0&t=6sThe term "hard water" refers to the concentration of mi.
hardness in water revision notes
Hard water can be softened by removing the calcium and magnesium ions Sometimes this can be achieved by boiling the water: . Water that can have its hardness removed by boiling it is called temporary hard water ; This method of removing hardness leads to the formation of scale which causes 'furring' of the heating element in a kettle making it less efficient
The test strips method is a simpler and quicker way to measure water hardness. The test strips are coated with a reactive material that changes color when exposed to calcium and magnesium ions. The user can then compare the color of the strip to a chart to determine the level of hardness. This method is less accurate than titration but is .A GCSE quiz focused on hard and soft water, looking at the causes and methods used to soften hard water. Also, looks at water quality and treatment. Scheduled maintenance: March 23, 2024 from 11:00 PM to 12:00 AM
Hardness testing within the realm of materials testing. Today, hardness testing is one of the most widely used methods in mechanical materials testing, especially for metals. On the one hand, this test method can be used to find qualitative relations to other material properties (e.g., strength, stiffness, density) or to the material behavior under certain stresses (e.g., abrasion .
GCSE; CCEA; Chemical analysis - (CCEA) Test for water Most elements are rarely found in their pure form. They are found chemically combined with other elements in compounds. In this GCSE Chemistry revision video, Hazel explains the difference between hard and soft water, temporary and permanent hard water, and methods used to rem. > GCSE Revision Notes > GCSE Chemistry > Hard Water. Hard Water. by revisioncentre 19 April 2020 20 April 2020 GCSE Chemistry. Hard water does not produce as much lather as soft water when it is used with soap and also an insoluble solid called scum is left on the sides of the wash basin.Impurities tend to increase the boiling point of water, so impure water will boil at temperatures above 100 o C; Impurities tend to decrease the melting point of water, so impure water will melt at temperatures below 0 o C; Distilled water. Distilled water is water that has been heated to form a vapour, and then condensed back to a liquid
Study with Quizlet and memorise flashcards containing terms like Why is water hard?, Why is water soft?, name the advantages of having hard water and others. . GCSE Chemistry/Water/hard water. Flashcards; Learn; Test; Match; Q-Chat; Get a hint. Why is water hard? Hard water contains lots of dissolved magnesium ions and calcium ions which are .Study with Quizlet and memorise flashcards containing terms like What is hard water?, what does the calcium and/or magnesium ions produce, hard water to react soap with?, Why does hard water reduce the efficacy of heating systems and kettles? and others. . Hard Water Chemistry GCSE. Flashcards; Learn; Test; Match; Q-Chat; Get a hint. What is .Chemistry coursework: Hardness of water. Planning. Aim. To investigate the hardness of water in 5 different water solutions. Background knowledge about hard water. The hardness of water is caused by the presence of Calcium 2+ and magnesium 2+ ions in the water. These minerals in water can cause some everyday problems. Presentation outlining the key specification points for AQA GCSE Chemistry on the topic of water. Perfect for use as a lesson and concise presentation ensures students remember key points of information for the exam. . Water *Hard and soft water *Removing hardness *Water treatment *Water issues and the public 3\. Energy Calculations .
Moderately hard water: 50-150 mg/ltr; Hard water: 150-300 mg/ltr; Very hard water:>300 mg/dl; Hard water is not suitable for industrial use. But hard water is usually beneficial for drinking purposes. However, hardness caused by MgSO4 gives some serious health effects. So, the concentration of Mg++ should not exceed 50 mg/lt in drinking water. 7.Hard water contains dissolved calcium or magnesium compounds. This can ‘fur up’ kettles, boilers and pipes, which wastes energy and can be dangerous if the flow of water is impeded. The calcium ions and magnesium ions in hard water react with the soap to form scum, so more soap is needed to form a lather. Temporary hard water contains .GCSE; AQA; Water - AQA Required practical. All humans rely on safe drinking water. Salt can be removed from sea water to make it safe to drink. Waste water must be treated before being released .Having introduced hard water previously, in this video we discuss the different types of hard water and how we approach the problem of softening them. We als.
Resultado da 1 Boca Juniors x Racing onde assistir ao vivo; 2 Onde assistir Boca Juniors x Racing e horário: 3 Escalações prováveis Boca Juniors x .
testing hardness of water gcse|hard water chemistry gcse